Heartbeam: The Device
New pick
Disclaimer: I’m not an investment advisor. Nothing I have written in this article should be taken as investment advice. Everything I have written here could be inaccurate. Trust nothing you just read. I’m part of the Seeking Alpha Affiliate program which means I have a financial relationship with Seeking Alpha.
Mongolian short AD: THE LINK
Get a 7-day free trial and 30$ discount on your first year of Seeking Alpha Premium with my Affiliate link: AFFILIATE LINK
Heartbeam Basic info:
Source: Google
IPO late 2021 into a strong market for tech innovation stocks. The stock price has performed poorly since then due to delays for commercialization, delays for FDA permits, and the overall worse market for small-cap innovation stocks.
Market cap=66m USD
No debt
No revenue at the moment. Target for revenue 2nd half of 2025.
Recently raised $11.5 million USD (at $1.70, no warrants, oversubscribed). This raise should sustain them for about a year.
The total addressable market exceeds $ 100 billion USD.
Heartbeam, the newest addition to The AlmostMongolian Portfolio, is my first healthcare stock and also my first heart attack-focused stock. Heartbeam has developed an innovative heart-monitoring device. The device is what this company and the investment thesis are all about, which means with this write-up, I will try to answer the following questions based on available information:
How does the device work?
What proof does Heartbeam have that the device works?
Is the device superior to the existing devices?
Is it permitted?
What is the plan for commercialization?
Who are the people owning and running Heartbeam?
In answering these questions, I have written the most comprehensive Heartbeam write-up on the internet.
To give you the basic idea of the thesis.
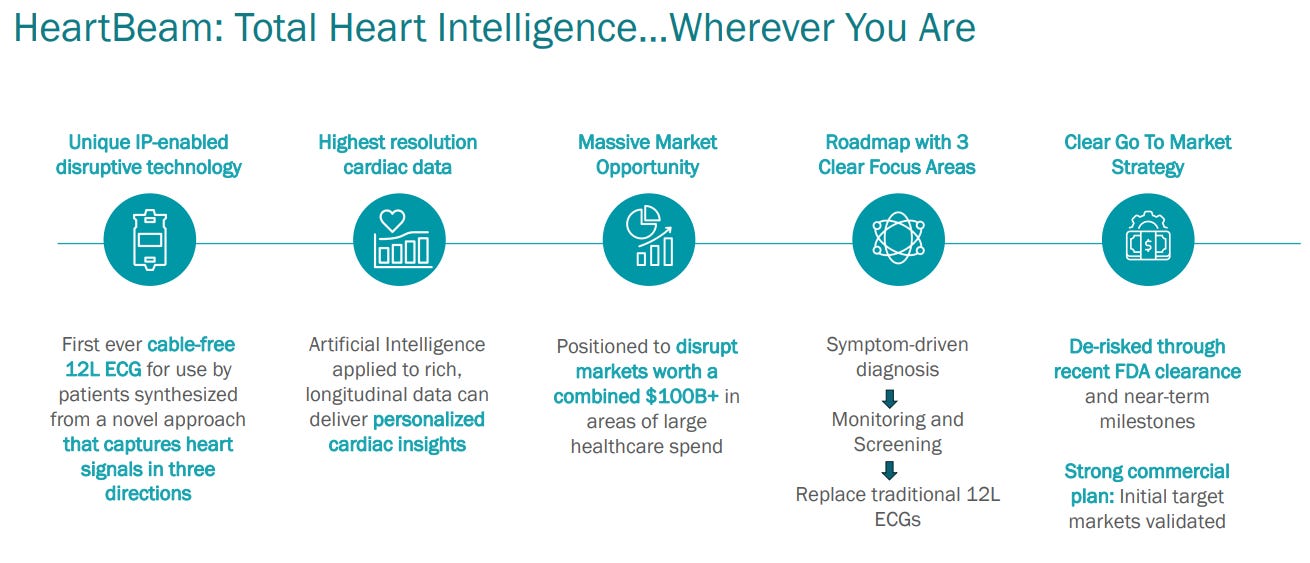
This is HeartBeam’s competition
Source: https://d1io3yog0oux5.cloudfront.net/_00cc34ec2f18b412abff79848819c77b/heartbeam/db/2293/21696/pdf/HeartBeam+investor+pres+03142025.pdf
The easy-to-use heart monitoring devices used at home and on the move are not accurate enough. Nobody who fears that their heart will brutally attack and murder them is solely going to rely on the Apple Watch. They will go to the hospital to obtain the best reading with a 12-lead ECG.
However, the current 12-lead ECGs are not practical for use at home or on the move due to the cables, size, and training required to use them properly. Just look at the standard 12-lead ECG on the right side of the picture. Imagine lying on a public beach or a park bench with that set-up, looking like you’re about to die any minute.
There is a demand in the market for a device that has hardware similar to the devices on the left side of the picture (easy to use, small) but with the capabilities of the device on the right side of the picture (accuracy).
This is what HeartBeam has developed: the first-ever cable-free 12-lead ECG system, approximately the size of a credit card.
Source for 2 slides above: https://d1io3yog0oux5.cloudfront.net/_00cc34ec2f18b412abff79848819c77b/heartbeam/db/2293/21696/pdf/HeartBeam+investor+pres+03142025.pdf
This shows the competitive situation perfectly. Of course, the top-right is where you want to be, and it’s only Heartbeam in there. I didn’t just take their word for it; I tried to look for competitors online, but I didn’t find any other devices in the market or in development that could be placed on the top right.
Source: https://d1io3yog0oux5.cloudfront.net/_00cc34ec2f18b412abff79848819c77b/heartbeam/db/2293/21696/pdf/HeartBeam+investor+pres+03142025.pdf
Quick detour. Here, it says 17 patents. Their March 2024 PR states that they hold 14 patents in the US and 4 internationally. Using my calculator, I calculated that this translates to a total of 18 patents. I don’t know what happened to that 1 patent.
But we don’t need to obsess over that 1 patent. Overall, they have a vast patent collection.
They are also looking to expand it. That same press release also states that they have 20 pending patent applications worldwide. The whole world.
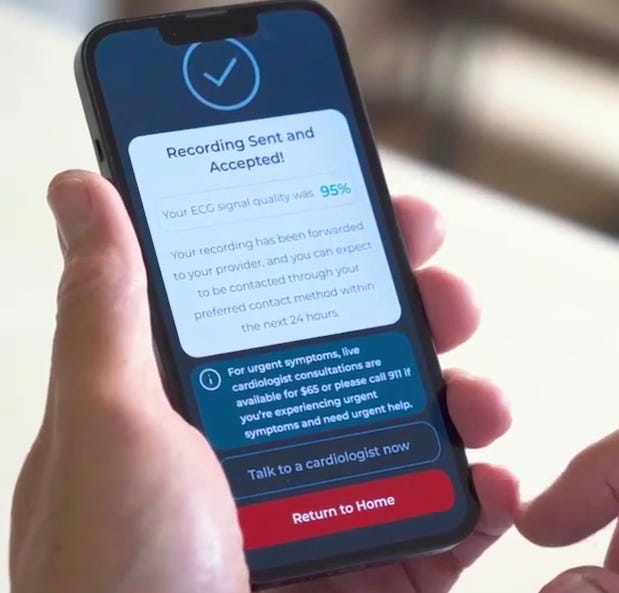
Here is the demo for the HeartBeams device. It’s very easy to use and fast to use.
Source: https://ir.heartbeam.com/
They have an app, which means recurring revenue. The average user will be old, so it’s crucial that the app is easy to use, and as you can see from the video, it is. You just put the device where your heart is (if you don’t know, the app will tell you) and press start recording. It takes 30 seconds to get the results. The results are sent to the patient's cardiologist, and within 24 hours, the patient should receive a response from the cardiologist.
The app also provides an option to speak with a cardiologist immediately for $65.
Source: https://ir.heartbeam.com/
The competition, which is the cabled 12-lead ECGs performed mostly in hospitals, takes 10-15 minutes, not accounting, of course, for the time it takes to go to the hospital.
Quick demand outlook:
We all know that our heart becomes our greatest enemy as we age. According to the World Health Organisation around 30% of global deaths are due to cardiovascular diseases. And it’s the leading cause of death globally. The population is getting old in rich countries(Heartbeam’s first target market). The demand for this kind of product is strong and will get stronger.
FDA approvals and submissions
Before commercialization, Heartbeam needs FDA clearance for its products.
They need two crucial FDA approvals. 1 for the hardware and 1 for the software.
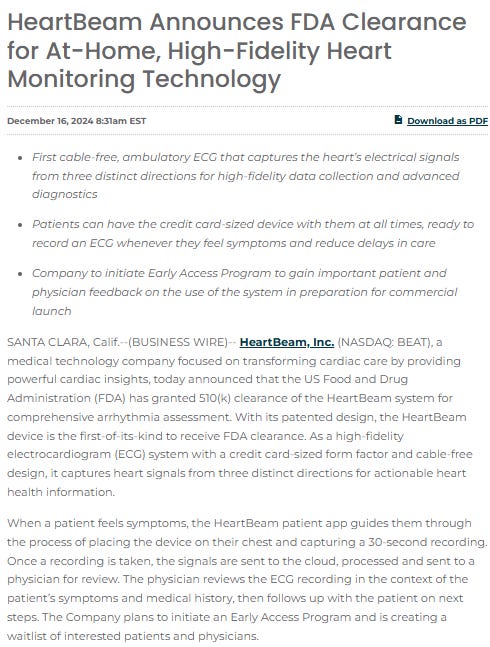
FDA approval for the hardware was granted in December 2024, and the FDA submission for the software was submitted in January 2025.
Source: https://ir.heartbeam.com/news-events/press-releases/detail/83/heartbeam-announces-fda-clearance-for-at-home
This is already very important because it serves as a crucial piece of validation for the technology. FDA 510(k) clearance means that the product is deemed safe and effective for its intended purpose.
The purpose, as stated in the PR, is a “comprehensive arrhythmia assessment.”
arrhythmia=abnormal heart rhythm
This FDA clearance also means the product can be sold. The question then arises as to why they are not selling it yet.
“Our first webcast question asks, congratulations on receiving their first FDA clearance, what went into your decision to not immediately commercialize upon clearance.
Rob Eno
Yes, I'll take that one. So just to recap, our initial clearance is for the system with the three-lead output and the second submission is the 12-lead Synthesis Software. And we just think it's important that we initially commercialize with the 12-lead software, which is really where we think the differentiation is from a physician and a patient perspective. So we're using this time after the initial clearance while waiting for that second clearance to fully prepare for commercialization with the Early Access Program. Hope that helps.” Q4 earnings call transcript
Source:https://d1io3yog0oux5.cloudfront.net/_00cc34ec2f18b412abff79848819c77b/heartbeam/db/2293/21696/pdf/HeartBeam+investor+pres+03142025.pdf
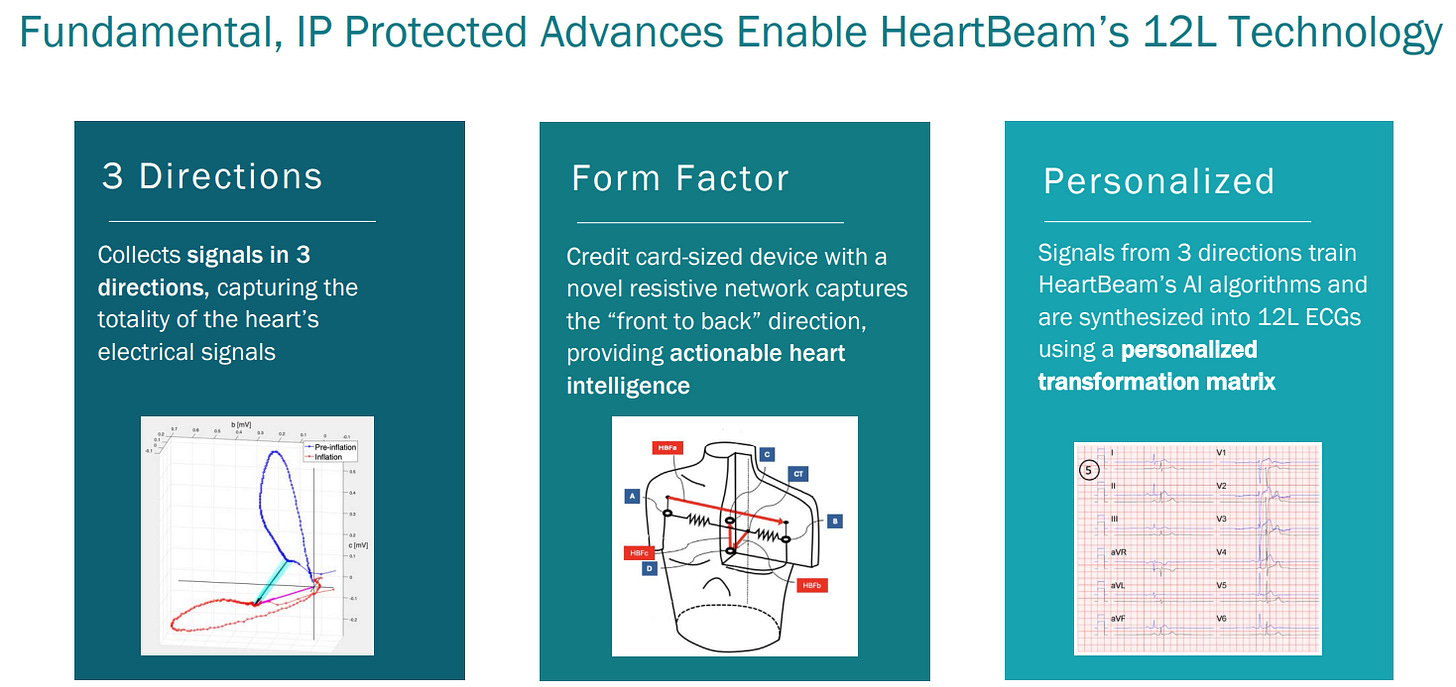
In summary, There are two pieces that both need FDA approvals for optimal commercialization.
FDA approved is the device that “collects signals in 3 directions, capturing the totality of heart’s electricity signals”
FDA submitted “Signals from 3 directions train HeartBeam’s AI algorithms and are synthesized into 12L ECGs using a personalized transformation matrix”
Source: https://ir.heartbeam.com/news-events/press-releases/detail/85/heartbeam-achieves-major-milestone-with-fda-submission-for
In terms of near- and mid-term catalysts, this second FDA clearance is the biggest one. It would be a big de-risking event, and If they obtain it, the company will be ready to proceed with full commercialization. Based on existing studies, the first FDA approval, and blind trust, I believe it’s highly likely that the software will receive FDA clearance; however, it’s always a possibility that it won’t, which is one of the risks associated with this stock.
When?
“Next question asked, when is the expected clearance time from FDA for the second clearance?
Rob Eno
Yes, it's always challenging and even in today's environment, particularly so in predicting the timing of FDA reviews and clearances. We're estimating that we'll receive the clearance before the end of the year, but right now, it's a little early for us to give any more specific guidance with that.
I'll just add that we have excellent relationships with FDA. We feel good about our submission. We held two pre-submission meetings with FDA to specifically talk about the design of the VALID-ECG study. And then as we've mentioned, we're encouraged by the results of the VALID-ECG study, which will be the main focus of the application and also those results will be presented publicly at the end of April.” Q4 call
They mention the VALID-ECG study and that they are encouraged by the results that will be presented at the end of April. This is likely our most near-term catalyst, as it is a more important study than usual because this study is a component of this FDA submission, which means if the results are positive, as they are indicating with ” encouraged,” the FDA clearance is highly likely. My confidence is further increased by the fact that they conducted a smaller study using the same protocol, which yielded good results.
I delve into this study further in the studies section of the article.
This was about 1/3 of the article. 2/3 are behind the paywall. The topics covered behind the paywall are commercialization strategy, scientific evidence, an interesting institution backing them, a summary, and some other things.
Want to keep reading without paying? Gain access to paid content by generating subscriptions for AlmostMongolian using the two buttons below.